Diagnosis of celiac disease is based on four elements. It is essential that patients follow a gluten-containing diet both before and during the diagnostic procedure.
The four key elements of a celiac disease diagnosis are:
- Clinical history
- Serology
- Histology
- Improvement of symptoms and antibody response to a gluten-free diet
1. Clinical history
Clinical history includes both individual and family medical/diet history. The diet history should include typical eating patterns, cultural traditions, and food preferences. Clinical symptoms are also assessed in this step, including bowel habits, weight loss, abdominal bloating, abdominal pain, nausea, and growth disorders in children. Extraintestinal symptoms such as fatigue, skin manifestations bone/joint pain, and headaches/migraine are also important factors to consider.
2. Serological tests
Serological testing for celiac disease involves the identification of immunoglobulin A tissue transglutaminase (IgA tTG) antibodies and specific endomysial antibodies (EMA). Total IgA should be measured in order to rule out IgA deficiency and reduce the risk of a false negative result. In patients with known IgA deficiency, immunoglobulin G (IgG) EMA, IgG deaminated gliadin peptide (DGP) or IgG tTG may be used in order to support the diagnosis [1,2]. It is essential that patients consume a gluten containing diet prior to and during diagnostic investigation to avoid a potential false negative result.
HLA genotyping
Human leukocyte antigen (HLA) DQ2/DQ8 testing may be considered as part of the diagnostic work-up. The diagnostic value of HLA genotyping revolves around it’s high negative predictive value – a negative result indicating that a patient is highly unlikely to have celiac disease (less than 1% of patients with celiac disease do not carry these alleles [3]). However, the positive predictive value of value of HLA genotyping is very low, since up to 40% of the general population also carry genes encoding HLA DQ2/DQ8 [4]. Therefore, HLA testing may be useful for patients where the diagnosis is ambiguous, or for those who have already embarked on a gluten free diet and choose not to undergo a gluten challenge.
In 2019, The European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) published updated guidelines for diagnosing celiac disease in the pediatric population. Genetic testing is no longer required in children with high antibody levels. The updated guideline states that for children whose blood tests show a level of IgA tissue transglutaminase (tTG) >10 x the upper limit of normal, a biopsy may not be needed to confirm a diagnosis. However, a positive endomysial antibody (EMA) test is required to confirm diagnosis in a no-biopsy approach. For children with IgA deficiency a no-biopsy approach should not be offered [10].
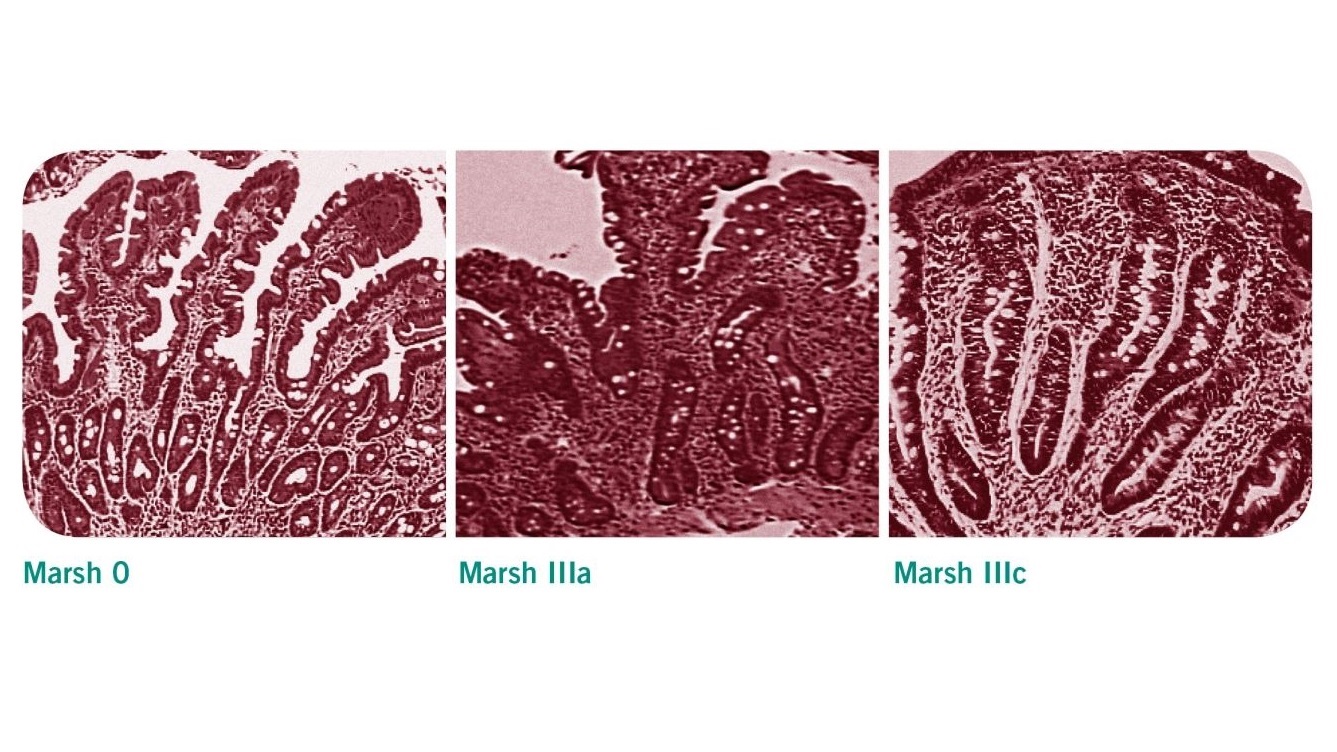
3. Histological tests
Patients with positive serological test results should be referred to a gastrointestinal for endoscopic intestinal biopsy to confirm or exclude celiac disease [2]. Villous atrophy may be patchy in celiac disease, therefore a minimum of four biopsy specimens should be obtained, including a duodenal bulb biopsy [1]. The histological changes observed in these samples are classified according to the Marsh classification. Evidence of villous atrophy and increased intraepithelial lymphocytes are typical features of a celiac-positive histology (Marsh 3a-c).
According to ESPGHAN guidelines, a no-biopsy approach can be offered to children with high levels of antibodies but who are asymptomatic. This approach can be adopted based on shared decision making between parents/child and the healthcare professional team [10].
| Marsh classification | histological findings |
|---|---|
| Stage 0 | Normal duodenal mucosa |
| Stage 1 | Increased intraepithelial lymphocytes (IELs) >25 IELs/100 enterocytes (non-specific finding) |
| Stage 2 | Stage 1 plus crypt hyperplasia (non-specific finding) |
| Stage 3a | Increased IELs, crypt hyperplasia, and partial villous atrophy |
| Stage 3b | Increased IELs, crypt hyperplasia, and subtotal villous atrophy |
| Stage 3c | Increased IELs, crypt hyperplasia, and total villous atrophy |
5. Response to a gluten-free diet
The diagnosis of celiac disease is considered to be confirmed if symptoms improve and repeat serological testing indicates that the antibodies are responding to the gluten-free diet. Current NICE guidelines on the Recognition, Assessment and Management of Celiac Disease [2] recommend that patients with persistently high serological titres or persistent symptoms after 12 months (where the possibility of continued gluten exposure has been excluded) should be considered for repeat intestinal biopsy and review by a specialist gastroenterology team.
Regular follow-up examinations
Guidelines from the Primary Care Society for Gastroenterology (PCSG) [8] recommend that newly diagnosed patients should be re-assessed after 3-6 months, and annually thereafter, in order to monitor compliance and response to the diet. Patients should also receive specialized nutrition counseling with a dietitian, during which symptoms may be reviewed alongside dietary adherence, nutritional adequacy, and collection of anthropometrical data [2]. Repeat serological testing may be used in conjunction with diet assessment to assess adherence. Follow-up biopsies are not routinely used in the review of patients with celiac disease, but may be useful for patients whose condition does not respond to a gluten free diet [1].
References
- Ludvigsson JF, Bai JC, Biagi F et al. Diagnosis and management of adult coeliac disease : guidelines from the British Society of Gastroenterology. Gut 2014; 63(8):1210-28
- NICE Clinical Guideline 86: Recognition, Assessment & Management of Coeliac Disease. National Institute of Clinical Excellence 2015.
- Polvi A, Arranz E, Fernandez-Arquero M et al. HLA-DQ2-negative celiac disease in Finland and Spain. Hum Immunol 1998;59:169-75
- Abadie V, Sollid LM, Barreiro LB et al. Integration of genetic and immunological insights into a model of celiac disease pathogenesis. Annu Rev Immunol 2011; 29:493-525
- Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol 1999; 11:1185-94
- Husby S, Koletzko S, Korponay-Szabó IR et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012; 54: 136-160
- Evaluation of the ESPGHAN Celiac Guidelines in a North American Pediatric Population. Gidrewicz D, Potter K, Trevenen CL, Lyon M, Butzner JD. Am J Gastroenterol. 2015 May;110(5):760-7. doi: 10.1038/ajg.2015.87.
- Fasano, A., Catassi, C. (2012). Clinical practice. Celiac disease. N Engl J Med.; 20; 367(25):2419-26. doi: 10.1056/NEJMcp1113994.
- Celiac disease: past, present, and future challenges: dedicated to the memory of our friend and colleague, Prof David Branski (1944-2013). Heyman MB. J Pediatr Gastroenterol Nutr. 2014 Jul;59 Suppl 1:S1. doi:10.1097/01.mpg.0000450390.38403.22
- Husby, Steffen; Koletzko, Sibylle; Korponay-Szabó, Ilma; Kurppa, Kalle; Mearin, Maria Luisa||; Ribes-Koninckx, Carmen; Shamir, Raanan; Troncone, Riccardo; Auricchio, Renata; Castillejo, Gemma; Christensen, Robin; Dolinsek, Jernej; Gillett, Peter||||; Hróbjartsson, Asbjørn; Koltai, Tunde; Maki, Markku; Nielsen, Sabrina Mai; Popp, Alina; Størdal, Ketil; Werkstetter, Katharina; Wessels, Margreet. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020, Journal of Pediatric Gastroenterology and Nutrition: January 2020 - Volume 70 - Issue 1 - p 141-156 doi: 10.1097/MPG.0000000000002497

